In chronic migraine, botulinum toxin reduces migraine frequency by 2 days/month and has a favorable safety profile (Herd et al, 2019).
The doses and injection techniques in the earlier reports were variable, and so were the results. It was until the Phase III Research Evaluating Migraine Prophylaxis Therapy (PREEMPT) 1 and 2 studies when its efficacy and safety, as well as the indication, i.e., chronic migraine (CM), were ascertained. (wang, 2020)
Long-term, real-world preventive treatment of CM with onabotulinumtoxinA showed effectiveness with a sustained reduction in headache-day frequency and significant improvement in quality-of-life measures. Adverse effects were mild to moderate. (Ahmed et al, 2019).
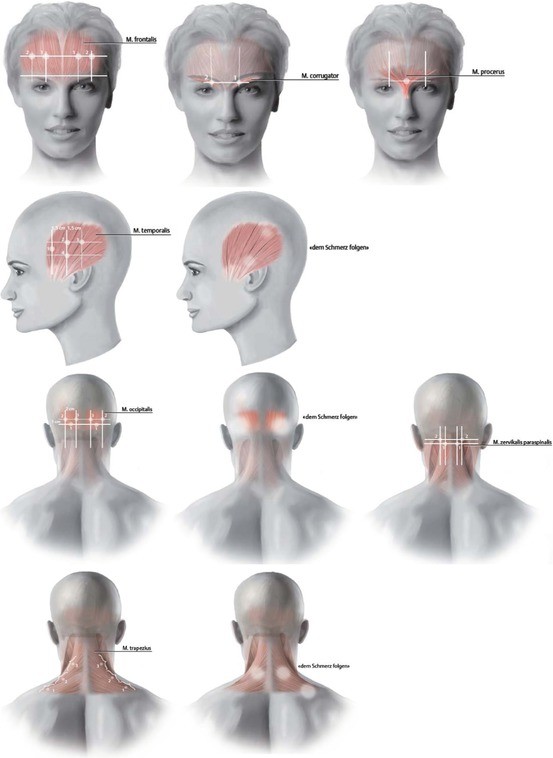
¶ Injection sites for migraine protocol (Agosti, 2015)
¶ Reference
Bendtsen, L., Sacco, S., Ashina, M. et al. Guideline on the use of onabotulinumtoxinA in chronic migraine: a consensus statement from the European Headache Federation. J Headache Pain 19, 91 (2018). https://doi.org/10.1186/s10194-018-0921-8
Herd CP, Tomlinson CL, Rick C, Scotton WJ, Edwards J, Ives NJ, Clarke CE, Sinclair AJ. Cochrane systematic review and meta-analysis of botulinum toxin for the prevention of migraine. BMJ Open. 2019 Jul 16;9(7):e027953. doi: 10.1136/bmjopen-2018-027953. PMID: 31315864; PMCID: PMC6661560.
Wang YF. Onabotulinumtoxin A injection in the treatment of chronic migraine. Prog Brain Res. 2020;255:171-206. doi: 10.1016/bs.pbr.2020.05.013. Epub 2020 Jun 30. PMID: 33008506.
Ahmed F, Gaul C, García-Moncó JC, Sommer K, Martelletti P; REPOSE Principal Investigators. An open-label prospective study of the real-life use of onabotulinumtoxinA for the treatment of chronic migraine: the REPOSE study. J Headache Pain. 2019 Mar 7;20(1):26. doi: 10.1186/s10194-019-0976-1. PMID: 30845917; PMCID: PMC6734221.
Agosti R. (2015) Refractory Chronic Migraine Therapy with Botulinum Toxin A. In: Siva A., Lampl C. (eds) Case-Based Diagnosis and Management of Headache Disorders. Headache. Springer, Cham. https://doi.org/10.1007/978-3-319-06886-2_6